The following publication has been lightly reedited for spelling, grammar, and style to provide better searchability and an improved reading experience. No substantive changes impacting the data, analysis, or conclusions have been made. A PDF of the originally published version is available here.
U.S. farmers have been using advances in technology to increase output and improve productivity for generations. Well-known sources of productivity gains in agriculture include increased mechanization, improved plant and animal nutrition, and the use of pesticides to control insects and weeds. Especially important has been improved plant and livestock genetics, particularly the development of plant hybrids that bring higher yields. These changes have provided extraordinary benefits to U.S. consumers in the form of a nutritious, high-quality, and inexpensive food supply.
Recent technological advances in agriculture have come in the area of genetic engineering, sometimes referred to as biotechnology, which has often been cited as holding the potential to provide tremendous benefits to production agriculture, processors, and consumers. For example, plant breeders may use biotechnology to increase crop yields, reducing the pressure to cultivate environmentally fragile land as global population rises. In addition, the enhanced ability to withstand climatic extremes permits crop production in areas where the land is not fragile, but the climate is unfavorable. Furthermore, improved plant resistance to insects and weeds allows farmers to reduce chemical use and the related environmental impact, while increasing the practicality of reduced tillage and conservation practices that depend on chemical weed control.
According to the U.S. Department of Agriculture (USDA), the use of biotechnology to incorporate value-added traits that improve the nutritional content or reduce processing costs of farm commodities is becoming increasingly important. For example, bioengineered high oleic soybeans contain less saturated fat than conventional soybeans and require less processing. Colored cotton reduces the need for chemical dyes. Nutraceuticals include crops designed to produce medicine or meet special nutritional needs. This category is especially important for poor countries where an adequate, nutritious diet is not readily available for large segments of the population. Some commodities have been modified to be more resistant to spoilage, reducing physical and economic losses, which helps hold the line on both manufacturing costs and retail prices. Finally, many believe that the future of U.S. agriculture lies in its ability to export food and commodities that meet the needs and wants of consumers around the world. The ability to improve quality and uniformity while tailoring products to specific groups of foreign consumers is a powerful competitive tool that enhances the ability of the U.S. food sector to compete in world markets.
Despite these benefits, controversy surrounding the use of agricultural biotechnology has escalated in recent months. Critics contend the methods used to modify crops have gone too far and safeguards are too loose, and also question the potential effects on the environment and the safety of related food products. In response to the rising level of apprehension, several processors and retailers in Europe announced they would discontinue using crops or selling products containing genetically modified organisms (GMOs). Some firms in the U.S. followed suit, despite statements from the U.S. Food and Drug Administration (FDA) that GMO crops are not significantly different from conventional crops. In addition, last fall a large U.S. grain processor asked its suppliers to keep GMO grain separate from conventional grain. Finally, European nations have required labeling for food containing GMO crops and have also expressed a reluctance to allow imports of GMO corn and soybeans from the U.S.
Since grain containing GMOs forms a growing segment of American agriculture, these actions hold enormous implications for U.S. farmers, as well as the farm input supply, processing, and transportation industries. Data from the USDA suggest that approximately one-third of the corn produced in the U.S. consists of varieties that were modified using biotechnology, while over half of domestic soybean production falls into this category. Several other nations, including Canada and Argentina, also have a large stake in the use of agricultural biotechnology. This Chicago Fed Letter provides some background on the use of biotechnology to improve crops, describes the concerns regarding its use, and discusses two related issues that have arisen in recent months—food labeling and grain segregation.
What is biotechnology?
Broadly defined, biotechnology includes any technique that uses living organisms to make or modify products, improve plants or animals, or develop microorganisms for specific uses.1 Scientists are able to isolate and transfer specific genes across organisms and can improve crops by introducing into a plant a copy of a gene for a desired trait, such as resistance to drought or disease. Since genes may be copied from any organism (plant, animal, microbe), plant breeders now have access to a much broader source of potentially useful genes and traits than would be available through conventional breeding methods. Furthermore, this control over specific genes and traits overcomes two primary disadvantages of conventional plant breeding techniques, namely, the transference of undesirable traits and the lengthy time required to breed these traits out of future generations of plants.
Some nonagricultural uses of biotechnology include insulin production and drugs that reduce the symptoms of arthritis and help prevent rejection of transplanted kidneys. The first commercial application of agricultural biotechnology approved by the FDA was the production of chymosin, an agent used in cheese production. The first genetically modified whole food to be approved by the FDA and marketed was the Flavr Savr tomato in 1994, which was developed to stay firm after harvest and could ripen on the vine for better flavor. More recent applications of biotechnology, and a source of current controversy, include corn varieties engineered to resist insect damage and soybeans that are unaffected by a popular herbicide. Both products were first marketed in 1996 and their use increased rapidly. Bt corn is a product designed to be resistant to the European corn borer, an insect that is responsible for a great deal of damage to corn crops across the Midwest. Farmers were quick to adopt Bt corn since it controlled the corn borer while reducing the need for manufactured insecticides. In comparison, soybean varieties were developed to withstand a broad-spectrum herbicide, glyphosate, which is effective in controlling both broadleaf weeds and grasses. Since it can be applied after the crop has emerged, it affords farmers more flexibility in weed control than preemergent herbicides.
Regulation
The FDA is responsible for the safety of domestic and imported foods in the U.S., except for red meat and poultry, which are regulated by the USDA. The Environmental Protection Agency (EPA) is the primary regulator for pesticides, while the FDA also monitors and enforces the tolerances set by the EPA. The FDA requires extensive premarket testing and approval for genetically modified crops if they are substantially different in structure and function from the conventional varieties already in our food supply. Labeling of food products containing these crops is required only if they undergo substantial transformation or could cause an allergic reaction. For example, Bt corn and herbicide-resistant soybeans did not meet the requirement for FDA premarket testing or labeling, as they were not deemed to be significantly different from corn and soybean varieties that already had a history of safe use. Nonetheless, the FDA regularly consults with firms developing new products to evaluate food safety concerns.
Why the concern now?
With all the benefits, why have biotechnology and genetically enhanced crops now become an issue? In general, the current controversy seems to stem from three factors. First, there are concerns over the safety of these crops for human consumption. Second, questions have been raised about the potential impact on the environment. Third, food scares unrelated to biotechnology have raised public awareness regarding the safety of our food supply and allowed biotechnology to become a larger issue.
Food safety
Critics contend that transferring a gene from a plant or organism that contains an allergen may cause the receiving plant to produce the allergen as well and claim that FDA procedures to evaluate this risk are inadequate. A similar complaint is made about naturally occurring plant toxins. There is also concern that the use of special gene markers may give rise to microbes resistant to antibiotics.
Environmental safety
The primary environmental concern is that genetically enhanced plants may pollinate related plants and allow the genes that are responsible for herbicide resistance or insect resistance to “escape” into the wild, leading to weeds and insects that are immune to modern chemicals. There is also a fear that nontarget insects may suffer damage from insect-resistant plants such as Bt corn.
Food scares
Though unrelated to biotechnology, some recent food scares have helped intensify concern about safety issues, especially in Europe. BSE (mad cow disease) in the United Kingdom and foot and mouth disease in Taiwan resulted in the authorities having to destroy a large portion of the livestock herds in those two nations. The discovery of livestock feed contaminated with dioxins (a cause of cancer) in Europe last year also raised the level of concern. The U.S. recently experienced an episode of meat contaminated with the listeria bacteria.
Supply chain issues
To date the primary concerns over the use of GMO seed have been registered in Europe and Japan. Responding to these concerns, some processors and retailers announced they will not use GMO crops in their food products or will label products regarding the GMO content. These actions raised several issues in the supply chain regarding segregation of GMO and conventional seed, identity preservation of commodities, testing for the presence of GMOs, acceptable tolerances, and questions of liability if commodities are falsely represented as being free of GMOs.
Segregation of crops
Responding to concerns that foreign buyers might refuse to accept grain or oilseeds containing GMOs, a large U.S. grain processor announced in late 1999 that it was asking suppliers to keep GMO grain separate from conventional grain. The problem today is one of infrastructure, i.e., most farmers, handlers, and processors are not prepared to segregate grain. Doing so requires either considerable downtime during harvest or processing for cleaning equipment so that mixing does not occur, or the purchase of additional equipment that could be dedicated solely to specific crop varieties. Either approach entails a significant additional cost.
Complete segregation may be all but impossible. Mixing can occur at several levels. First, even conventional seed may contain low levels of GMOs. Second, mixing of genetic material is possible through cross-pollination of crops that are planted in the same or adjacent fields. Indeed, farmers are encouraged to plant strips of conventional corn in fields with Bt corn to provide a “preserve” for nonresistant corn borer insects that can breed with those that may be immune to the Bt protein, thus passing along susceptibility. Finally, mixing may occur if GMO crops and conventional crops are run through the same equipment that is not thoroughly cleaned between loads.
Testing, standards, and liability
Since GMO grain cannot be distinguished from conventional grain by visual inspection, testing procedures must be established to determine GMO content at different points in the supply chain. Testing kits were not readily available last fall, so first-level buyers were forced to rely on farmers to certify the grain was free of GMOs. Testing kits will likely be readily available at harvest time in the fall of 2000. But the issue of mixing underscores the legal question of who bears the responsibility if a load of grain that is represented as GMO-free is found to contain GMOs. Furthermore, it has already been noted that complete segregation may be impossible. Consequently, standards must be established for acceptable levels of GMOs in grain and oilseeds, perhaps similar to standards already in existence for levels of foreign matter allowed in various commodities.
Labeling of food products
Critics of agricultural biotechnology have essentially adopted a three-pronged attack: first, demanding the federal government ban the use of GMOs; second, asking the FDA to regulate GMOs more heavily; and third, recasting the issue as one of consumer choice and asking the FDA to require labeling of all food products containing GMOs. At present, the third goal seems the most likely to become reality, given the decision to label GMO food products in Europe and Japan and the support expressed by some members of the U.S. Congress for this type of food labeling. The type of label then becomes an issue.2 “Positive labels” such as “This product may contain GMOs” impart little to no information that would allow a consumer to make an informed decision; whereas, “This product contains no GMOs” allows consumers to distinguish between products, allowing a clear choice and perhaps furthering the development of niche markets.
Implications
Farmers are advised to keep crops containing GMOs separate from conventional varieties and to avoid mixing. Though the pricing implications are not yet well established, this would enable them to take advantage of any price premiums associated with conventional crops or avoid discounts associated with mixed grain. Of course, this assumes that farmers will continue to voluntarily plant seed developed through modern biotechnology. Many may decide to reduce the amount of GMO seed planted because of concern over price discounts and demand uncertainty. This would have serious implications for firms that have invested heavily in the new technology for developing GMO seed. Furthermore, segregation may increase unit costs within the U.S. food system, making smaller and/or higher-cost firms less viable and encouraging industry consolidation. Cost increases would eventually be reflected at the retail level.
Some firms could be in a position to benefit from grain segregation. Farmers that provide harvesting services to other farmers, and are willing to dedicate harvest and transportation equipment to either GMO or conventional varieties, could experience increased demand for their services. Some farmers may choose to purchase additional harvesting equipment and build additional storage facilities to segregate grain. Farm equipment manufacturers and some construction firms would benefit from the duplication of equipment and facilities, at least in the short run. Grain handlers and processors may also need to gear up with additional equipment to handle segregation. Some grain elevators may specialize in either conventional or GMO crops. And to the extent that additional investment in equipment is necessary, financing opportunities would be available to commercial banks and other lenders. Finally, firms that provide testing services or equipment to establish the presence of GMOs at different points in the supply chain could experience strong demand for their products.
Conclusion
Biotechnology and genetically enhanced crops hold great potential to reduce costs, improve the environment, and contribute to human health goals. However, concerns over the safety of these foods and the environmental impact of genetically enhanced crops have risen dramatically in recent months. Though it seems highly unlikely that the continued development and use of these products will be banned, labeling and perhaps additional regulation may increase costs in the food system and, ultimately, affect retail food prices. The type of labeling would have a significant impact on consumers’ ability to make informed choices and the establishment of segmented or niche markets for food containing GMOs and food from conventional crops.
Tracking Midwest manufacturing activity
Manufacturing output indexes (1992=100)
| October | Month ago | Year ago | |
|---|---|---|---|
| CFMMI | 135.4 | 134.8 | 130.9 |
| IP | 143.4 | 142.6 | 138.3 |
Motor vehicle production (millions, seasonally adj. annual rate)
| October | Month ago | Year ago | |
|---|---|---|---|
| Cars | 5.7 | 5.7 | 6.1 |
| Light trucks | 7.1 | 6.9 | 6.6 |
Purchasing managers' surveys: net % reporting production growth
| November | Month ago | Year ago | |
|---|---|---|---|
| MW | 61.6 | 60.4 | 54.2 |
| U.S. | 57.4 | 58.3 | 48.5 |
Purchasing managers' surveys (production index)
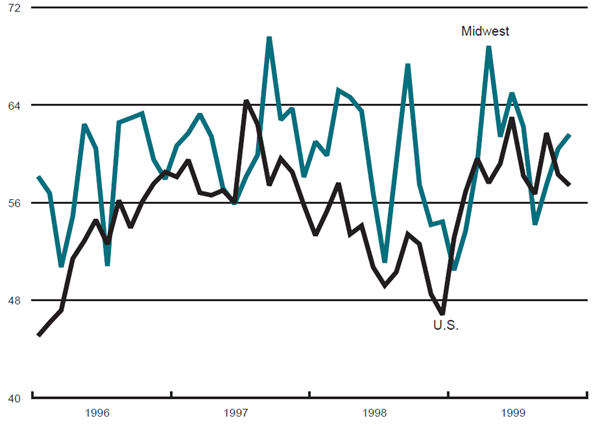
The Midwest purchasing managers’ composite index (a weighted average of the Chicago, Detroit, and Milwaukee surveys) for production increased from 60.4% in October to 61.6% in November. The purchasing manager’s indexes increased slightly for Detroit and Milwaukee but declined for the Chicago index. The national purchasing managers’ survey for production decreased from 58.3% to 57.4% from October to November.
Light truck production increased from 6.9 million units in September to 7.1 million units in October, and car production remained constant at 5.7 million units for both September and October. The Chicago Fed Midwest Manufacturing Index (CFMMI) increased 0.5% from September to October. Revised data show the index fell 0.2% in September. The national Industrial Production Index for manufacturing (IP) also increased at a rate of 0.6% for the same period.
Notes
1 U.S. Congress, Office of Technology Assessment, 1992, A New Technological Era for American Agriculture, Washington, DC: U.S. Government Printing Office, No. OTA-F-474, August.
2 C. Ford Runge and Lee Ann Jackson, 1999, “Labeling, trade, and genetically modified organisms (GMOs): A proposed solution,” University of Minnesota, Center for International Food and Agricultural Policy, working paper, No. WP99–4.










